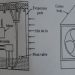
*Evaporative coolerslower the temperature of air using the principle of evaporative cooling.
*Evaporative cooling is the addition of water vapour into air, which causes a lowering of the temperature of the air.
*The energy needed to evaporate the water is taken from the air in the form of sensible heat, which affects the temperature of the air, and converted into latent heat.
*This conversion of sensible heat to latent heat is known as an adiabatic process because it occurs at a constant enthalpy value.
*Evaporative cooling therefore causes a drop in the temperature of air proportional to the sensible heat drop and an increase in humidity proportional to the latent gain.
*A simple example of natural evaporative cooling is perspiration, or sweat, secreted by the body, evaporation of which cools the body.
*The amount of heat transfer depends on the evaporation rate, however for each kilogram of water vaporized 2,257 kJ of energy transferred area.
*The evaporation rate depends on the temperature and humidity of the air, which is why sweat accumulates more on hot, humid days, as it does not evaporate fast enough.
*Vapour compression refrigeration uses evaporative cooling, but the evaporated vapour is within a sealed system and is then compressed ready to evaporate again, using energy to do so.
*A simple evaporative cooler water is evaporated into the environment, and not recovered.
*In an interior space cooling unit, the evaporated water is introduced into the space along with the now-cooled air, in an evaporative tower the evaporated water is carried off in the airflow exhaust.